We’re here to help you, your staff, and families living with CPP every step of the way
Triptodur CareSM Resources:
Enroll your patients today
Download these resources to get started:
Patient Enrollment Form (English)
Patient Enrollment Form (Spanish)
Triptodur Care Program Summary
Request Additional Materials and Support Services
Copay Program Portal HCP User Guide
Copay Program Enrollment and Reimbursement Process
In-Home Nurse Injection Program Enrollment Form
Administration Resources
These resources will help you understand the full process of reconstitution and administration prior to injection

Download the Triptodur Administration Guide for full information on reconstitution and administration, including helpful tips.
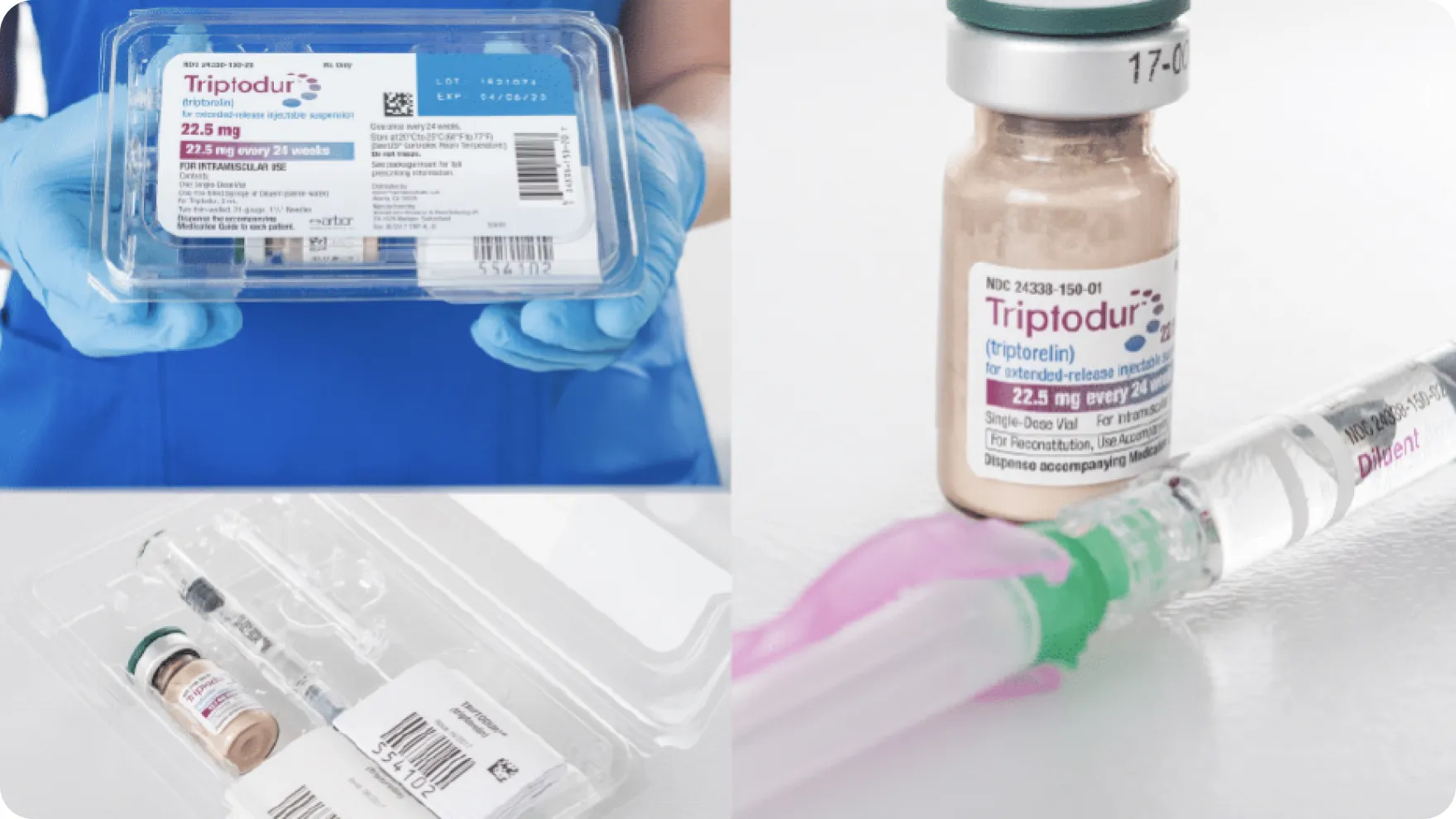
Watch the Triptodur Administration Video, which provides step-by-step instructions for reconstitution and administration in an easy-to-follow visual format.


Request virtual or live in-service support for Triptodur administration by completing this form.
Patient and Caregiver Resources
Meet Trip the Dino. Trip is here to support you and your child on your journey with Triptodur, along with additional helpful materials.

Triptodur Patient Welcome Kit that includes Trip the Dino, a supportive stuffed animal, Trip and Growing Up Ahead of Schedule Storybook, and caregiver educational materials all delivered in a Triptodur bag.

Video Resources

Trip and Growing Up Ahead of Schedule

For Children: Trip the Dinosaur was examined by a doctor and diagnosed with CPP

For Parents and Caregivers: Learn more about Triptodur® (triptorelin) for treating CPP
Medical Billing Resources
Download these forms for coding and billing guidance to avoid potential issues before they happen.
Checklist for Prior Authorization Submission
Support and Materials Request
If you need anything else, complete our Materials and Support Services Request Form.
PP-TRIP-US-1269


